
OF has 14 valence electrons, four in the 2p orbitals (see the diagram in the answer to Problem 5.9).
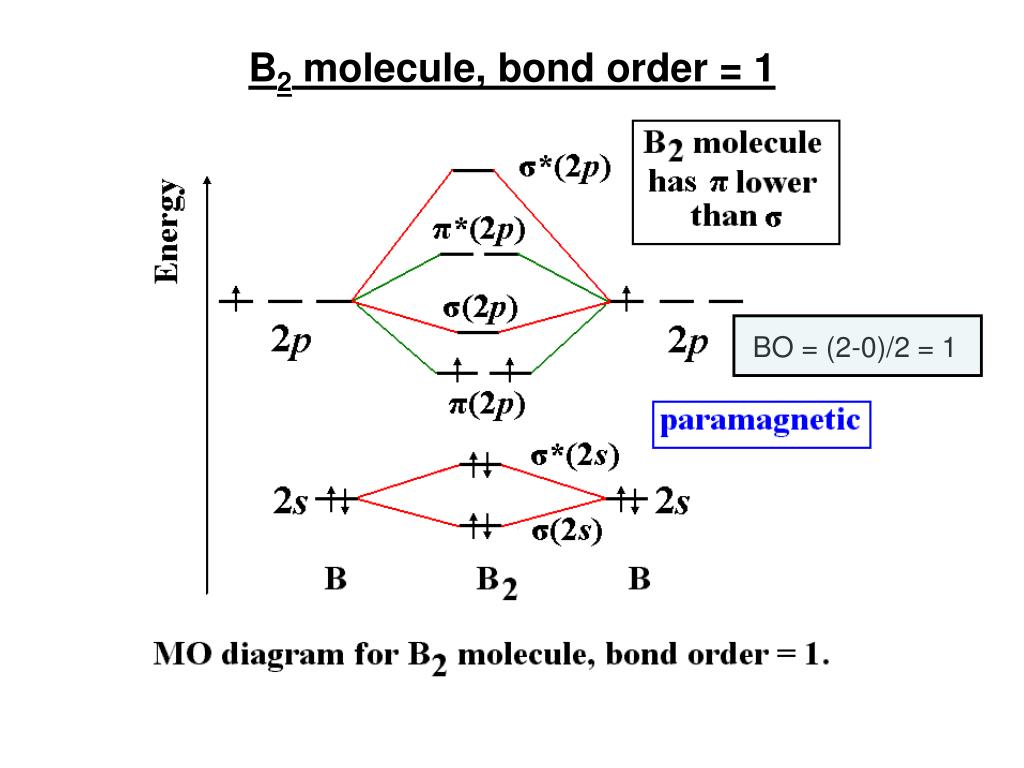
The 2s orbitals will overlap to form 2s and 2s. Each boron atom has one 2s and three 2p valence orbitals. We can ignore the 1s orbitals, because they do not contain the valence electrons. Then we rank them in order of increasing energy. A molecular orbital is a space where a specific pair of electrons are most likely to exist. The latter do not possess C2 rotation axes coincident to the infinite-fold rotation axis of the orbitals on the basis of the change in wave function sign upon crossing the nodes on the bond axis. Before we can draw a molecular orbital diagram for B, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. What type of molec- ular orbital contains the unpaired electron Is this. The basis of molecular orbital theory is the molecular orbitals produced from quantum mechanics.

The side-by-side overlap of two p orbitals gives rise to a pi ( ) bonding molecular orbital and a \ ( \) antibonding molecular orbital, as shown in Figure 8.4.5 8.4. The higher the molecular orbital bond order, the less stable the species. 4: Combining wave functions of two p atomic orbitals along the internuclear axis creates two molecular orbitals, p and p p. An increase in the number of antibonding electrons will cause the molecular orbital bond order to increase.

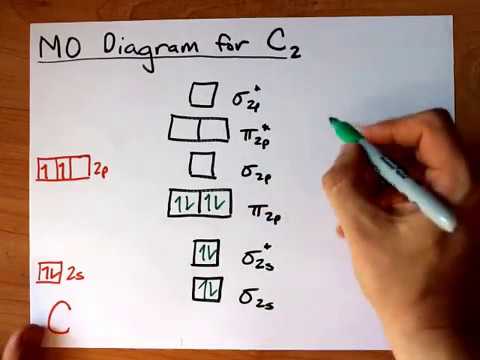
Each non-bonding pair is distributed over both oxygen atoms at once in molecular orbital theory, while in Lewis theory each lone pair is isolated to one atom or to localized bonds attached to that atom. The 1g (left panel) and 1u (right panel) molecular orbitals of C2 The next two molecular orbitals (2g and 2u) corresponds to the symmetric and, re-spectively, antisymmetric linear combinations of the 2s orbitals on each C atom. molecular - orbital theory, using only the 2p oxygen and the 1s hydrogen orbitals. The lower the molecular orbital bond order, the weaker the bonds between the atoms of the species. Still, notice that each orbital is spread across both oxygen atoms at once, and again we see that each non-bonding electron pair in the HOMO is very different in molecular orbital theory compared to Lewis theory. \): Two atomic orbitals (left) will overlap to form bonding (b) and antibonding (ab) molecular orbitals.\( \newcommand\) molecular orbitals, which are truly non-bonding and mostly oxygen in character.


 0 kommentar(er)
0 kommentar(er)
